Abstract
Introduction: BCP-ALL associated with t(5;14)(q31;q32) is an exceptional cause of eosinophilia. The IL3 gene expression becomes regulated by the IGH enhancer leading to an overproduction of IL3 causing eosinophilia. Clinical, biological and outcome data are extremely scarce in the literature.
Methods: We recruited 2 newly diagnosed patients (pts) with t(5;14)(q31;q32) in our centers. A retrospective search in the French protocol FRALLE 2000 data base and collaboration with the GFCH (Groupe Francophone de Cytogénétique Hématologique) added 4 other pts. Array CGH, MLPA or genomic PCR search for IKZF1 deletion were performed in these 6 pts. We further analysed the 12 pts with t(5;14)(q31;q32) described in the literature with our 6 newly described pts, leading to a meaningful series of 18 pts.
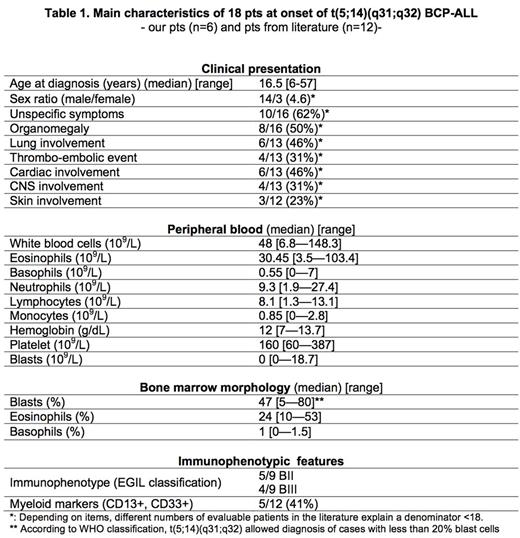
Results: Main features at onset are displayed in table 1. Median age at diagnosis is 16.5 years. Male/female ratio is 4.6. Eosinophilia-related symptoms are common with neurologic, thromboembolic or lung involvement (respectively 4/13, 4/13 and 6/13 pts). No pt exhibits blast cells in CSF at diagnosis. Median WBC count is high (48x109/L), mainly due to a high rate of eosinophils (median: 30.5x109/L). Blood eosinophilia preceded ALL diagnosis from 6 and 12 months in 2 cases. Peripheral blast cells are low or undetectable (median: 0x109/L; range: 0-18.7x109/L). A high basophilia is frequent (median: 0.55x109/L). Bone marrow morphology is marked by a low blast infiltration (median: 47%) and increased eosinophil-lineage cells (median: 24%) with dysplastic features in 6/10 pts. All cases are of the B lineage. All 18 pts but one present the t(5;14)(q31;q32) translocation. For the remaining pt, it was revealed by sequencing as karyotype was normal in a context of eosinophilia. Six out of 18 pts have additional cytogenetic abnormalities. For the first time, we report an IKZF1 deletion which is present in 4 out of 6 pts. Few other deletions are found in more than one pt (PAX5, ETV6, MEF2C, CDKN2A). Remission and outcome data are available for 11 pts (our 6 newly described pts and 5 from the literature). Median follow-up (FU) at point date is 18 months (range: 4 days-30 months). Only 5 pts achieve a first complete remission (CR1) at first attempt. The 6 newly described pts are currently alive: 4 are in continuous CR1 (CCR1) including 2 after hematopoietic stem cell transplantation (HSCT) and 2 are in CCR2 (including 1 after HSCT). The 5 pts from the literature died, 1 from cardiac arrest secondary to eosinophilia and the 4 others from disease progression and / or sepsis.
Discussion and Conclusion : 1) t(5;14)(q31;q32)-associated BCP-ALL has remarkable features including some which are shared with other IGH BCP-ALL, according to data from a comprehensive UK retrospective study using IGH FISH screening1. Both conditions affect preferentially teenagers and young adults (median age: 16 years1). The highly biased male to female ratio reported here is milder in other IGH BCP-ALL (1.441). In both entities a low blast count (<50 G/L in 119/158 pts1) is observed. Our new data revealed a high rate of IKZF1 deletion in 4/6 pts (66%) also observed in 35/88 pts (40%) in IGH BCP-ALL1. Differences obviously include the eosinophilia and related symptoms but also the low rate of cytopenias possibly linked to the partial bone marrow infiltration, prevailing in t(5;14)(q31;q32)-associated BCP-ALL. 2) In pediatric IGH BCP-ALL pts, 5-year overall survival is 84%1. The lower published survival rate in t(5;14)(q31;q32)-associated BCP-ALL should be balanced by a 100% survival in CR in our 6 newly described pts with obviously a limited sample size and a shorter FU. This may reflect new treatment strategies, including pediatric intensive chemotherapy and MRD-based stratification. HSCT seems to be an option for pts with suboptimal response (induction failure, high MRD) or relapse. 3) Despite being a specific WHO recognized entity, many common clinical and biological features allow to suggest that t(5;14)(q31;q32)-associated BCP-ALL may be considered as a very rare subset of IGH BCP-ALL. Nevertheless depending on its kinetics and severity, the specific eosinophilia can render difficult both diagnosis and management.
1. Russell LJ, Enshaei A, Jones L, et al. IGH@ translocations are prevalent in teenagers and young adults with acute lymphoblastic leukemia and are associated with a poor outcome. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014;32(14):1453-1462.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal